If you’re deciding between EEG, MEG, and fMRI, the core distinction is the signal each modality captures and the trade-off between temporal and spatial resolution. EEG and MEG record the brain’s electromagnetic activity directly, giving millisecond timing but coarser localization.
fMRI tracks hemodynamic changes (BOLD contrast) that follow neural activity, delivering millimeter-scale maps but with seconds-level delay.
Everything else, hardware, cost, artifacts, contraindications – flows from that physics.
Key points
- EEG measures scalp voltages from synchronous postsynaptic currents; ms timing, lower spatial precision, highly portable and inexpensive.
- MEG measures femtotesla magnetic fields from the same neuronal currents; ms timing and better source localization than EEG, but costly and infrastructure-heavy.
- fMRI measures the hemodynamic (BOLD) response; mm spatial maps, seconds-level lag, excellent for networks and deep structures.
- Choose EEG/MEG for fast dynamics (oscillations, ERPs/ERFs, spikes); choose fMRI for anatomy-aligned mapping (language, vision, resting-state networks).
- Combining them exploits EEG/MEG timing + fMRI spatial detail for the most complete picture.
What each modality actually measures (the essentials)
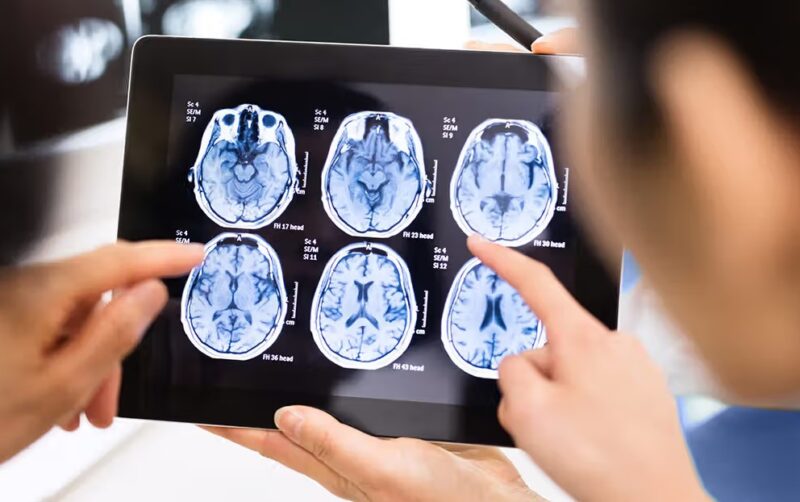
Neural populations generate electrical currents; these currents produce both voltages and magnetic fields, and—via neurovascular coupling—metabolic changes in blood oxygenation. That’s the full chain: currents → fields/voltages → hemodynamics. Each technique samples a different link, with distinct noise sources and modeling assumptions.
1. EEG ─ voltage at the scalp from cortical postsynaptic currents
Electroencephalography records potential differences across electrodes. It is maximally sensitive to radial sources (currents oriented perpendicular to cortex) and is strongly influenced by skull/scalp conductivity.
2. MEG ─ magnetic fields generated by the same neuronal currents
Magnetoencephalography uses SQUIDs or OPMs to sense tangential cortical currents. Magnetic fields are less distorted by skull conductivity, improving forward models and source localization.
3. fMRI ─ hemodynamic proxy of neural activity
Blood-oxygen-level–dependent contrast tracks local changes in deoxyhemoglobin driven by neural activity. The hemodynamic response function (HRF) peaks ~4–6 s after a brief burst of activity.
EEG ─ strengths, limits, and when it’s best

EEG is the workhorse for temporal dynamics. With high-density caps (64–256 channels), you can capture oscillatory phenomena (delta→gamma), event-related potentials (ERPs), and transient epileptiform discharges at the millisecond scale.
EEG isn’t only for labs. It underpins biofeedback and training scenarios that translate well outside clinics. For example, some programs deliver supervised neurofeedback at home, leveraging real-time EEG metrics to reinforce desired oscillatory patterns. While not a replacement for diagnostic studies, it shows how accessible the modality is for longitudinal monitoring and training.
Strengths
EEG’s temporal resolution is unmatched for price and portability. It tolerates typical environments, integrates with behavioral tasks, and supports long recordings (sleep, ambulatory monitoring). Artifact correction (blink/EMG/EKG) is mature, and inverse modeling pipelines (e.g., distributed source estimates) are widely available.
Limitations
Spatial localization is ill-posed: skull inhomogeneities smear potentials, and deep or gyral-tangential sources are harder to recover. Motion and muscle activity contaminate high-frequency bands; strict prep (impedances, gel) matters. Absolute depth sensitivity is limited.
Best use cases
- Epilepsy screening and spike detection; seizure onset timing.
- Cognitive paradigms that hinge on timing (perception, attention, error monitoring).
- Sleep staging, anesthesia depth, and bedside neurophysiology.
MEG ─ when magnetic fields help
MEG measures the same neuronal generators as EEG but via magnetic fields, using SQUID cryogenic sensors or newer optically pumped magnetometers (OPMs). Recordings occur in magnetically shielded rooms to suppress environmental noise.
Strengths
Because magnetic fields are less distorted by skull/scalp, MEG enables more accurate source localization, particularly for tangential sources in sulci. You keep millisecond temporal fidelity and can map oscillatory networks and evoked responses (ERFs) with improved spatial precision relative to EEG.
Limitations
MEG is expensive, infrastructure-intensive, and sensitive to subject motion (especially with SQUID helmets). It is less sensitive to deep sources (e.g., hippocampus) and still requires head models and co-registration with MRI for source reconstruction.
Best use cases
- Pre-surgical mapping of eloquent cortex; interictal spike localization.
- Oscillatory network dynamics with precise timing and better spatial priors.
- Developmental and sensory processing studies where motion-tolerant OPM setups help.
fMRI ─ hemodynamic mapping at scale
fMRI aligns neural function to anatomy with strong spatial fidelity. The BOLD signal arises from neurovascular coupling—changes in cerebral blood flow/volume and oxygenation—producing high-contrast maps at 2–3 mm (or finer with high-field scanners).
Strengths
Spatial resolution is the headline: whole-brain coverage, deep structures, and network-level inference. Task fMRI cleanly localizes sensory, motor, and language cortices; resting-state fMRI identifies intrinsic functional networks (DMN, attention, salience). Non-invasive and widely available.
Limitations
The HRF introduces a seconds-long lag and blurs rapid events; vascular variability (age, medication, pathology) can confound neural interpretations. Motion, susceptibility, and physiological noise (respiration, cardiac) demand rigorous preprocessing. MR safety and claustrophobia are practical constraints.
Best use cases
- Surgical planning (language lateralization, motor mapping).
- Systems-level network mapping and deep-structure involvement.
- Longitudinal studies of plasticity where anatomical alignment matters most.
Which one should you choose?
Start with the question you need to answer: Do I need timing at the millisecond scale, or do I need anatomical precision and deep coverage? Then factor in patient population, safety, and logistics. Questions to ask before booking:
- Is the phenomenon fast (e.g., oscillations, ERPs) or spatially specific (e.g., V1 retinotopy, language areas)?
- Are there implants or MR contraindications? If yes, EEG/MEG may be preferable.
- Do you need bedside/portable monitoring or whole-brain mapping?
- What’s the budget and local infrastructure (shielded room, MR scanner availability)?
Can they be combined?
The most informative designs pair modalities: EEG-fMRI acquires scalp voltages inside the scanner to link BOLD changes to specific electrophysiological events; MEG-fMRI uses co-registration and joint inverse modeling to embed millisecond timing into millimeter maps.
Data-fusion methods (e.g., joint ICA, Bayesian priors) exploit the complementarity: EEG/MEG constrain when, fMRI constrains where.
Practical multimodal examples
- Spike-triggered fMRI to localize epileptogenic zones.
- Visual or auditory tasks where MEG defines sequence timing and fMRI delineates cortical fields.
- Resting-state analyses comparing spectral power (EEG/MEG) with BOLD networks.
Bottom line
EEG and MEG are direct measures of neuronal electrophysiology with millisecond precision; fMRI is an indirect hemodynamic proxy with millimeter spatial detail.
For timing-critical questions, pick EEG or MEG (MEG if localization precision justifies the cost). For anatomy-anchored questions, pick fMRI.
When stakes and budget allow, combine them to avoid choosing between time and space.